Bananas ripening, cars rusting, food cooking, fires burning, volcanoes erupting, baking soda reacting with vinegar, milk souring, and metals tarnishing are some most common examples of chemical change.
- Examples of Chemical Change
- 1: Rusting Iron
- 2: Burning Wood
- 3: Cooking an Egg
- 4: Digesting Food
- 5: Ripening a Banana
- 6: Mixing Baking Soda and Vinegar
- 7: Burning Natural Gas
- 8: Developing Photos
- 9: Fireworks Exploding
- 10: Making Slime
- 11: Electroplating jewelry
- 12: Metal rusting
- 13: Milk souring
- 14: Baking bread
- 15: Changing hair color
- 16: Decomposing food
- 17: Photosynthesis
- 18: Cutting onions
- 19: Discoloring a sliced apple
Examples of Chemical Change
Here are the examples with some more detail added:
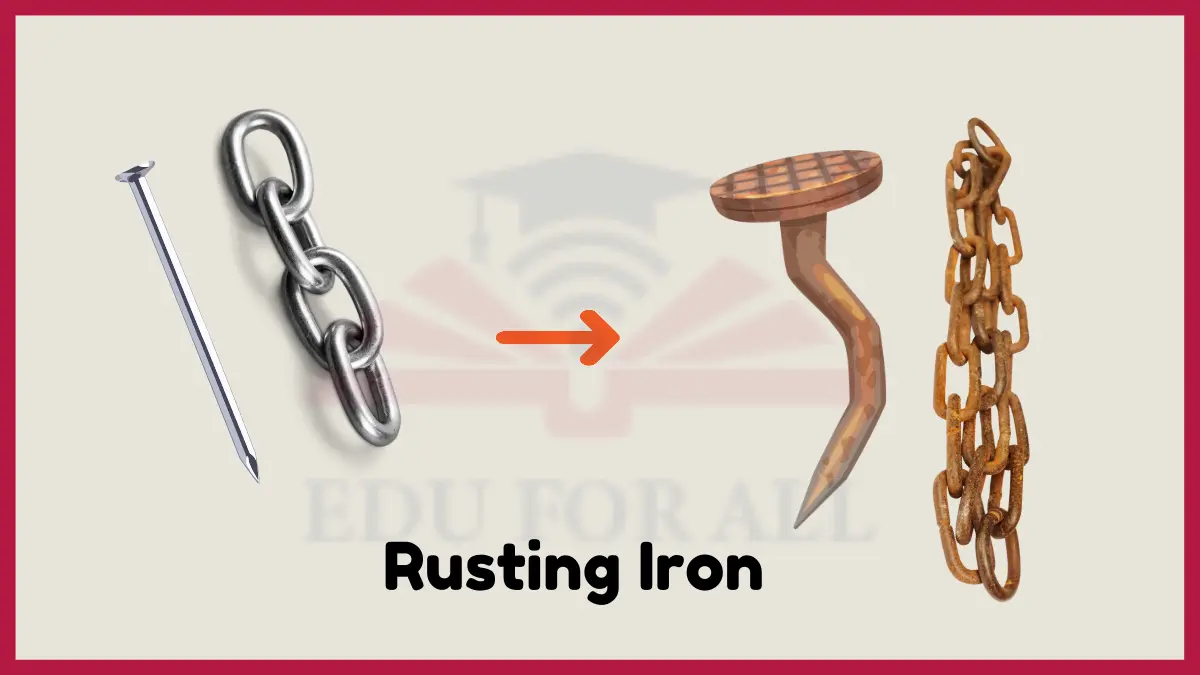
1: Rusting Iron
Iron rusts when it is exposed to oxygen. The iron reacts with oxygen to form iron oxide, which we see as the reddish-brown rust. This change happens faster when the iron is also touching water, because the water helps the oxygen react with the metal.
To experiment with iron rusting, you can leave a piece of iron outside in the rain or water and observe the formation of rust over time.
Rusting transforms hard, shiny iron into a crumbly, weak material that flakes off. This transformation is a chemical change because the iron has changed into a completely different substance called iron oxide.
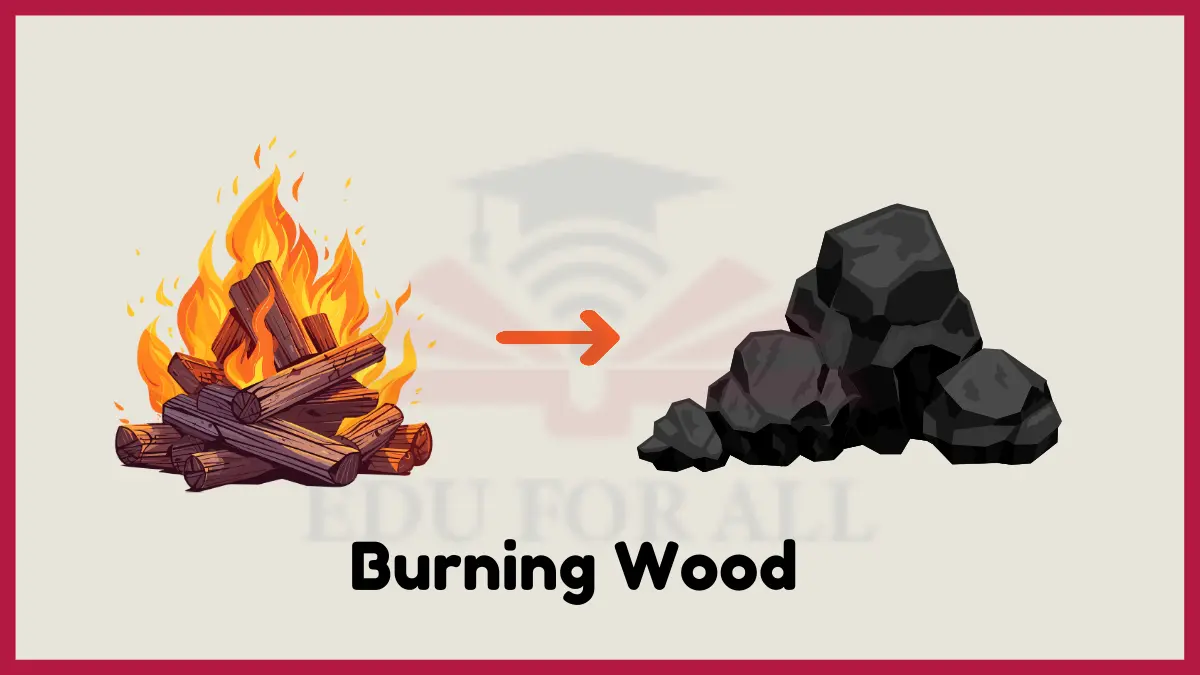
2: Burning Wood
When wood burns in a campfire or fireplace, it undergoes a chemical change called combustion. The heat of the fire ignites the wood and triggers chemical reactions with oxygen from the air. As the wood burns, it reacts with the oxygen to form new chemicals like carbon dioxide, water vapor, ash, and light energy.
To experiment with the combustion of wood, you can light a piece of wood on fire and observe the chemical changes as it burns, producing heat, light, and smoke.
Burning changes the brown solid wood into gases, smoke, and grey ash. You can no longer recognize the original wood after it has been burned, which shows combustion is a chemical change.
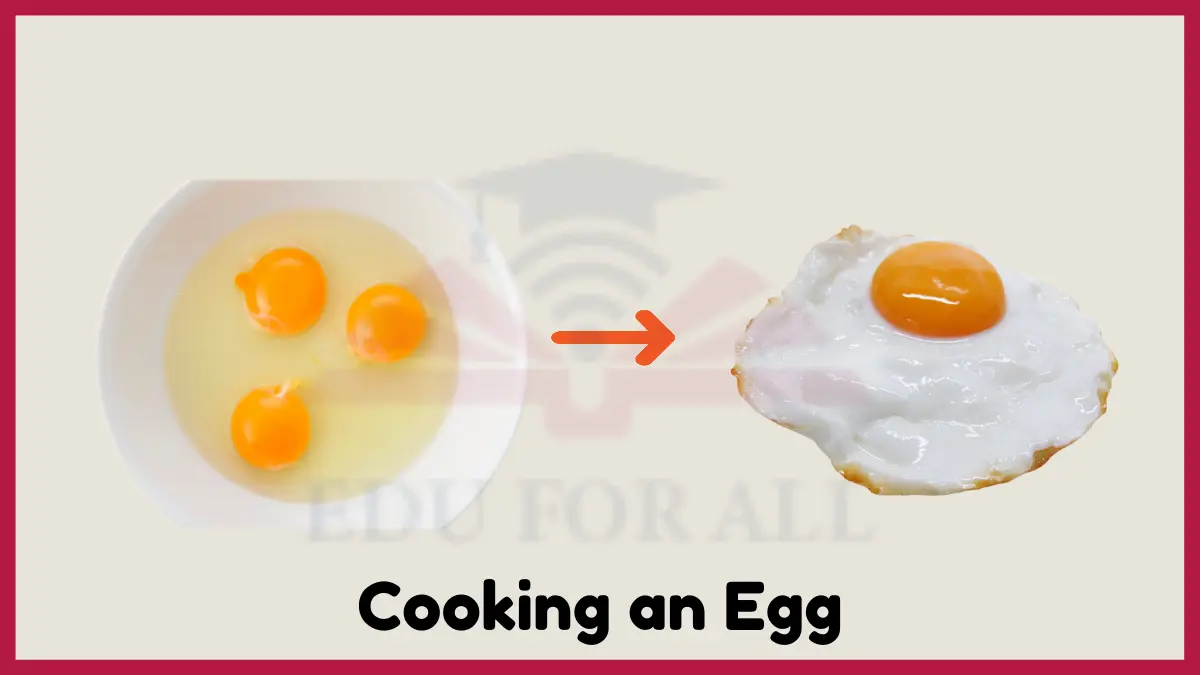
3: Cooking an Egg
Cooking an egg causes multiple chemical changes to occur. When an egg is heated, the clear liquid egg white undergoes a change and becomes opaque and white-colored. This is because the heat denatures the proteins in the eggs whites, causing them to unravel and then form new bonds with each other. The new bonds change the texture from runny to rubbery and firm.
Additionally, the yolk changes from a thick liquid to a semi-solid as heat causes the fats and proteins to harden. Overall, cooking chemically transforms the eggs from a translucent liquid to an opaque white and yellow solid.
To experiment with the denaturation of proteins, you can cook an egg and observe the change in its texture and color from a runny liquid to a solid white and yellow mass.
4: Digesting Food
Digestion involves many chemical changes that start in your mouth and continue through your gastrointestinal tract. Chewing food mechanically breaks it down and mixes it with saliva. Enzymes in saliva begin chemically breaking down starches into sugars. In the stomach, acids and enzymes further break down food into nutrients.
The small intestine continues using enzymes from the pancreas, liver and intestinal wall to chemically break down carbohydrates, fats and proteins into molecules the body can absorb. Digestion turns the pizza and french fries you ate into usable sugars, amino acids and fatty acids needed for energy, growth and function. The complex chemical changes are necessary for the body to extract nutrients from food.
5: Ripening a Banana
As a banana ripens, chemical changes occur inside the fruit that change its color, texture and flavor. While green, bananas are hard and starchy due to their high content of pectin and starch. During ripening, the chlorophyll in the banana peel breaks down, causing the skin to change from green to yellow.
To experiment with the enzymatic breakdown of starch into sugars, you can observe the ripening process of a banana by leaving it at room temperature and noting the changes in its color, texture, and flavor as it ripens.
At the same time, enzymes convert starch into sugars, making the banana softer and sweeter. By the end of the ripening process, the banana tastes much sweeter due to the increase in fructose and other sugars.
The changes in color, firmness and flavor demonstrate that bananas undergo chemical changes during the ripening process.

6: Mixing Baking Soda and Vinegar
When baking soda and vinegar are mixed together, an exciting fizzing reaction occurs. This is because acetic acid in the vinegar reacts with sodium bicarbonate (the chemical name for baking soda). The acid-base reaction produces carbon dioxide gas, which forms bubbles.
You can test this chemical reaction yourself by combining baking soda and vinegar in a bottle or balloon.
The production of a new substance (carbon dioxide) shows that a chemical change took place. The fizzing results from the gas being rapidly released during the reaction.
7: Burning Natural Gas
Burning natural gas is a chemical process that involves hydrocarbon fuels reacting with oxygen to produce new substances. Natural gas consists mostly of methane, along with some other hydrocarbons like propane and butane.
When methane combines with oxygen and burns, the chemical reaction releases energy and converts the methane and oxygen into carbon dioxide and water vapor.
You can observe these chemical products if you burn natural gas in a stove and notice water droplets forming on a cool surface.
The new gases formed demonstrate that burning natural gas causes chemical changes to occur.
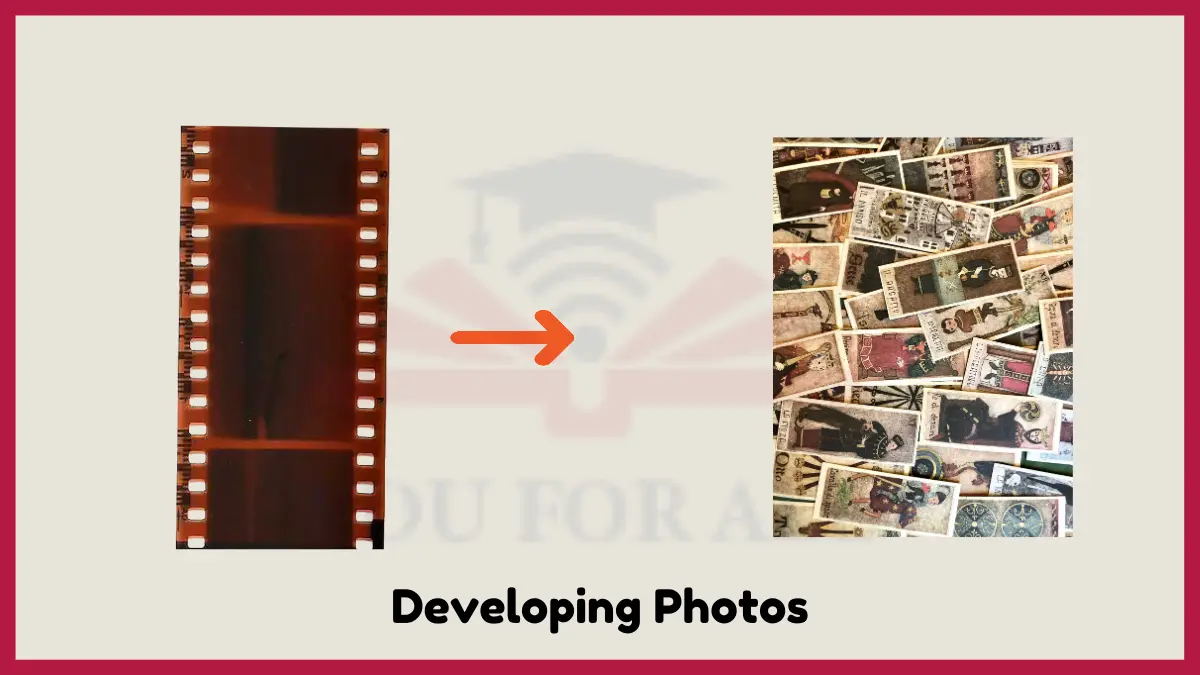
8: Developing Photos
Film photography relies on chemical changes induced by light to produce images. Photographic film contains many silver bromide crystals embedded on its surface. When light strikes the crystals, the silver ions change their arrangement, resulting in subtle chemical changes.
Later, developing solutions cause the affected silver ions to cluster into visible metallic silver specks that darken and form the image. The unexposed crystals remain unchanged. Without chemical reactions, the film would remain a blank slate. Photography provides a great example of how light can initiate chemical changes that form a permanent product.
To experiment with the light-sensitive chemical changes in photographic film, you can take a photograph and develop it using a developing kit, observing how the exposed silver halide crystals transform into visible silver specks forming the image.
9: Fireworks Exploding
The dazzling effects of fireworks rely on a wide range of chemical reactions that occur when the fireworks are ignited. Fuses are lit to ignite gunpowder propellants that launch fireworks into the sky. Additional chemical mixtures produce colored flames, flashes, and bursting effects. Strontium and copper compounds generate vivid red and blue colors when they undergo chemical decomposition and burn.
To experiment with the rapid chemical reactions in fireworks, you can light a firework and observe the dazzling display of colored flames, flashes, and bursting effects, all resulting from the combustion and decomposition of various chemical compounds.
Bangs and whistles result from combustion reactions that quickly release gases. Each visual and auditory effect depends on chemicals rapidly undergoing changes to emit light, sound, and pressure. Fireworks beautifully exemplify how combustion and decomposition can create spectacular sensory effects through chemical changes.

10: Making Slime
Popular slime recipes for kids often mix together solutions of borax with white school glue. The borax acts as a cross-linking agent that chemically binds to the polymer chains in the glue. This causes the individual polymers to link together into a flexible, stretchy network that exhibits properties of a solid.
To experiment with the cross-linking of polymer chains, you can mix together borax and glue and observe the formation of a sticky, elastic mass of slime due to the chemical bonds formed between the borax and the glue’s polymer chains.
The chemical reaction turns the slippery, flowing glue mixture into a sticky, elastic mass of slime that can be picked up and molded into shapes. The changes in viscosity and texture show that new chemical bonds formed, turning liquid glue into solid slime.
11: Electroplating jewelry
Electroplating applies a coating of metal onto jewelry and other products through an electrochemical process. The item is submerged in a solution containing a dissolved metal, like silver or gold. When an electric current is applied, metal ions from the solution deposit onto the item’s surface forming a thin metallic layer.
To experiment with the cross-linking of polymer chains, you can mix together borax and glue and observe the formation of a sticky, elastic mass of slime due to the chemical bonds formed between the borax and the glue’s polymer chains.
This is a chemical process in which the dissolved metal reacts at the item’s surface to bond with and coat the item. Electroplating transforms and enhances the item’s appearance through chemical changes at a molecular level.
12: Metal rusting
When iron metal is exposed to air and moisture, it undergoes chemical changes known as corrosion or rusting. The iron reacts with oxygen to form iron oxide, the chemical name for rust. This oxide coating is brittle and flakes off as more iron is exposed and reacts.
To experiment with the corrosion of metals, you can leave a piece of metal outside in the rain or water and observe the formation of rust or tarnish, a result of the chemical reactions between the metal and oxygen or other atmospheric elements.
Rusting transforms strong structural iron into a crumbly, weak substance. Other metals like copper and aluminum also experience chemical reactions called tarnishing when their surfaces react with air. These chemical changes weaken metal over time.
13: Milk souring
Milk undergoes a chemical change when it sours and curdles. Lactose, the sugar found in milk, gets converted to lactic acid by bacterial action. The increase in acidity chemically alters the proteins in the milk, causing them to tangle into solid curds.
At the same time, liquid whey separates from the curds. The overall increase in acid curdles the fluid milk into chunky solids floating in thin liquid – a dramatic chemical change. Sour milk has a distinct tart taste and thick texture caused by these chemical reactions.
To experiment with the lactic acid fermentation of milk, you can leave a carton of milk out at room temperature and observe the changes in its taste, texture, and smell as the milk sours and curdles due to the bacterial action converting lactose into lactic acid

14: Baking bread
Baking bread involves chemical reactions between flour, yeast and water. When yeast is combined with warm water and sugars, it undergoes fermentation. This produces carbon dioxide gas and alcohol as byproducts. The gas gets trapped in the elastic gluten network formed when flour and water are mixed. As the dough proof, the gas bubbles expand, making the bread rise.
Additional gas and steam is produced from heat during baking. This causes the dough to expand further and setup the bread’s spongy texture. Chemical reactions at every stage are responsible for baking light, fluffy bread.
To experiment with the fermentation of yeast and the chemical changes in dough, you can mix together flour, yeast, water, and salt to make bread dough, let it rise due to the carbon dioxide produced by fermentation, and then bake it in an oven to observe the formation of a light, fluffy bread.
15: Changing hair color
Permanent hair coloring works through a complex series of chemical reactions that alter the structure and color of hair. The first step usually involves bleach to strip away natural pigment and open up the cuticle. Developer containing hydrogen peroxide then penetrates and reacts within the hair shaft to form new colored compounds.
Additional reagents help the new pigments remain within the damaged cuticle layer. The products chemically break apart and reform different color pigments to dye hair a new permanent shade.
16: Decomposing food
Rotting food emits foul odors caused by chemical changes from decomposition. Bacteria and fungi release enzymes that catalyze reactions breaking down complex food molecules into simpler compounds. Fats turn into fatty acids and glycerol.
Proteins break down into unpleasant nitrogenous substances like ammonia, amines, and sulfides that smell. Sugars transform to acidic metabolites. These chemical changes make up the process of decay that returns nutrients in organic matter to the ecosystem.
17: Photosynthesis
Photosynthesis is the process by which plants use sunlight, water, and carbon dioxide to produce oxygen and glucose, a type of sugar. This process is a chemical change because it converts the reactants (sunlight, water, and carbon dioxide) into new products (oxygen and glucose).
18: Cutting onions
Freshly cut onions can bring tears to your eyes due to the chemical reactions they induce. Onions contain amino acids called lachrymators that are released when cells rupture from cutting. These acids instantly vaporize to form irritating sulfuric acid in the air that triggers a burning sensation.
To produce the tear-inducing effects, the lachrymators undergo chemical changes as enzyme action converts them into new sulfur-containing vapors. Next time you cry over onions, remember it’s due to the onion’s defense chemicals!
19: Discoloring a sliced apple
When an apple is sliced and left out, it quickly begins to brown as chemical reactions occur. Inside cells, enzymes called polyphenol oxidases are released and interact with phenolic compounds in the apple tissues. This enzyme-mediated oxidation turns the phenolics into brown pigments called melanins.
Over time, more cells are damaged, releasing more enzymes, speeding up melanin production and brown discoloration. The entire apple slice can become completely browned as the enzymatic chemical changes affect the entire surface.

