A few common examples of alkynes include ethyne (acetylene), propyne, and butyne. Ethyne has the simplest structure (C2H2) with a triple bond between two carbons while propyne (C3H4) and butyne (C4H6) have triple bonds with longer carbon chains. Other basic alkynes are 2-pentyne, 3-hexyne, and 1-heptyne, which have 5, 6, and 7 carbon chains respectively featuring highly reactive triple bonds.
The electron density around the carbon-carbon triple bonds causes alkynes to display increased reactivity compared to other hydrocarbons.
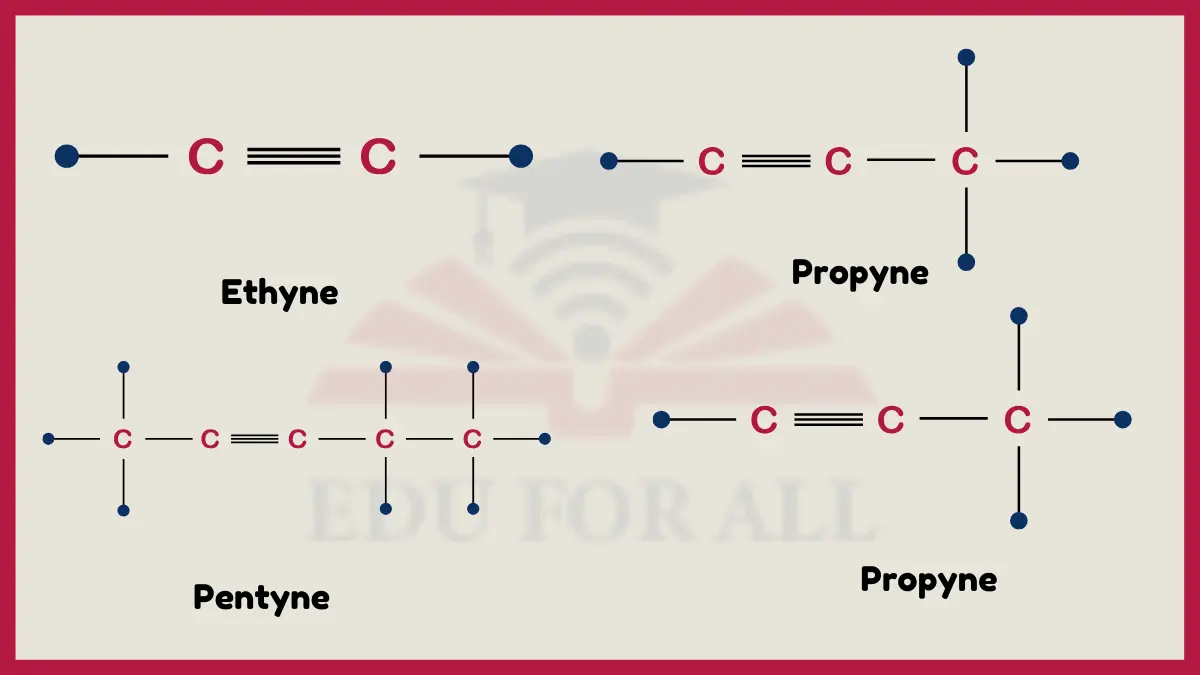
Examples of Alkynes
Here are 10 examples of alkynes:
1: Terminal Alkynes
General Formula: CnH2n-2 where n>=2
Terminal alkynes have a carbon-carbon triple bond at the end of the carbon chain. This bond consists of 1 sigma and 2 perpendicular pi bonds between two sp-hybridized carbon atoms.
Examples
- Ethyne (Acetylene): C2H2
- Propyne: CH≡C-CH3
- But-1-yne: CH≡C-CH2-CH3
- Pent-1-yne: CH≡C-CH2-CH2-CH3
- Hex-1-yne: CH≡C-(CH2)3-CH3
2: Symmetrical Internal Alkynes
General Formula: CnH2n-4 where n>=4
Symmetrical internal alkynes have a carbon-carbon triple bond between two internal carbons equidistant from the ends of the chain.
Examples
- Buta-2,3-diyne: CH≡C-C≡CH
- Penta-2,4-diyne: CH≡C-CH2-C≡CH
- Hepta-2,4-diyne: CH≡C-(CH2)2-C≡CH
- Nona-2,5-diyne: CH≡C-(CH2)3-C≡CH
- Undeca-3,6-diyne: CH≡C-(CH2)4-C≡CH
3: Asymmetrical Internal Alkynes
General Formula: R1-CnH2n-5-R2 where n>=3
Asymmetrical alkynes contain a triple bond located internally at unequal distances from the ends of the carbon chain.
Examples
- 3-Hexyne: CH≡C-CH2-CH2-CH3
- 5-Decyne: CH3-(CH2)3-C≡C-(CH2)4-CH3
- 2-Nonyne: CH3(CH2)6-C≡CH
- 4-Tetradecene: CH≡C-(CH2)11-CH3
- 7-Hexadecene: CH≡C-(CH2)13-CH3
4: Cycloalkynes
General Formula: CnH2n-2 where n>=3
Cycloalkynes contain a carbon-carbon triple bond within a saturated or unsaturated carbon ring structure.
Examples
- Cyclopropyne: c-C3H4
- Cyclohexyne: c-C6H8
- Cyclododecyne: c-C12H20
- 1,3,5-Cyclodecatriyne
5: Fluoroalkynes
General Formula: R1R2C≡CR3R4 where at least one R is F
Fluoroalkynes have at least one carbon substituted with a fluorine atom. The electronegativity affects reactivity.
Examples
- 3,3,3-Trifluoropropyne
- 4,4,4-Trifluorobutyne
- Perfluorobutyne
- 1-(Trifluoromethyl)-1-propyne
- 3,3,4,4,4-Pentafluorobutyne
6: Aryl Alkynes
General formula: Ar-C≡C-R
Aryl alkynes contain a carbon-carbon triple bond with at least one aryl group (benzene ring structure) attached.
Examples
- Phenylacetylene: C6H5-C≡CH
- 4-Ethynyltoluene: CH3-C6H4-C≡CH
- 3-Ethynylpyridine: NC5H4-C≡CH
- 1-Naphthylacetylene: C10H7-C≡CH
- 4-tert-Butylphenylacetylene: (CH3)3CC6H4-C≡CH
7: Haloalkynes
General formula: X-C≡C-R
Haloalkynes contain one or more halogen atoms (F, Cl, Br, I) substituted on a carbon of the carbon-carbon triple bond.
Examples
- Chloroacetylene: Cl-C≡CH
- Dichloroacetylene: Cl-C≡C-Cl
- 1-Chloropropyne: Cl-C≡C-CH3
- 4-Bromopentyne: Br-(CH2)2-C≡CH
- 1-Iodo-3-hexyne: I-CH2-CH2-CH2-C≡CH
8: Alkynols
General formula: RC≡CCH2OH
Alkynols feature a hydroxy group (-OH) on the carbon adjacent to the alkyne carbon-carbon triple bond.
Examples
- Propargyl alcohol: HC≡CCH2OH
- 1-Butyn-3-ol: CH3CH2C≡CCH2OH
- 3-Hexyn-1-ol: CH3(CH2)2C≡CCH2OH
- 1-Octyn-4-ol: CH3(CH2)5C≡CCH2OH
- 2-Nonyn-6-ol: CH3(CH2)4C≡CCH2CH2CH2CH2OH
9: Carbonyl Alkynes
General formula: RC≡COR
Carbonyl alkynes contain two alkyl or aryl groups attached to a carbon-carbon triple bond having a carbonyl group on one end.
Examples
- Propiolaldehyde: HC≡CCHO
- Hex-3-yn-2-one: CH3CH2CH2C≡C(C=O)CH3
- Phenyl propiolic acid: C6H5-C≡C-COOH
- Ethynyl p-tolyl ketone: CH3-C6H4-C≡C(C=O)-CH3
- 5-Undecynal: CH≡C-(CH2)8-CHO
10: Enynes
General Formula: R1C≡CR2-CH=CH2
Enynes contain a carbon-carbon triple bond conjugated with a C=C double bond. This alternation affects reactivity.
Examples
- 3,7-Octadiyne: HC≡C-CH=CH-C≡CH
- 1,3,7-Decatriene: CH≡CH-CH=CH-CH2-CH=CH2
- 5,9-Hexadecadiene: CH≡C-(CH2)7-CH=CH-(CH2)7-CH3
- 2-Buten-1-yne: CH≡C-CH=CHCH3
- 5-Undecene-8-yne: CH2=CH-(CH2)3C≡C(CH2)5CH3
11: Cumulenes
General Formula: R1C≡C-C≡CR2
Cumulenes contain two or more triple bonds in a carbon chain with no single bonds in between. This builds up angle strain.
Examples
- Butatriene: H-C≡C-C≡C-H
- Octatetrayne: HC≡C-C≡C-C≡C-C≡CH
- Dodecapentayne: HC≡C-C≡C-(CH2)3-C≡C-(CH2)3-C≡CH
- Diphenylbutadiyne: C6H5-C≡C-C≡C-C6H5
- Hexylheptatetrayne: CH3(CH2)5-C≡C-C≡C-(CH2)2-C≡C-C≡CH
12: Trialkynes
General Formula: (RC≡C)3CH
Trialkynes have an sp hybridized central carbon bound to three separate alkynyl groups in a tetrahedral geometry.
Examples
- Ethyl 3-(buta-1,3-diynyl)pent-2-ynoate
- Propane-1,1,1-triyltris(benzene-4,1-diyl) triacetate
- 4-(Prop-2-yn-1-yl)-6-(but-3-yn-1-yl)phenyl 4-(pent-4-yn-1-yl)benzoate
- 1,1,1-tris(phenylethynyl)ethane
- 5-(4-bromophenylethynyl)-10,15-bis(phenylethynyl)zinc porphyrin

