The mechanisms by which bleach disinfects, natural gas combusts, table salt dissolves, nitrous oxide reduces pain, titanium resists corrosion, polyester avoids wrinkles, vinegar removes lime deposits, liquids conform to containers, quartz glass maintains dimensions, and activated charcoal absorbs odors are examples of chemical properties.
- Examples of Chemical Properties
- 1. Bleach’s Ability to Disinfect
- 2. Natural Gas Combustibility
- 3. Table Salt’s Solubility
- 4. Nitrous Oxide’s Analgesic Properties
- 5. Titanium’s Resistivity to Corrosion
- 6. Polyester’s Wrinkle Resistance
- 7. Vinegar’s Ability to Dissolve Lime Deposits
- 8. Liquids Ability to Take Shape of Containers
- 9. Quartz Midpoint Thermal Expansion
- 10. Activated Charcoal Odor Absorption
Examples of Chemical Properties
Here are the 10 examples of chemical properties:
1. Bleach’s Ability to Disinfect
Bleach chemically dissolves microbe cell walls and destroys viruses and bacteria. This demonstrates the reactive chemical property of bleach to break down other compounds.
About 5.75 billion pounds of bleach are produced annually in the United States for cleaning and disinfecting.

Experiment: Add a few drops of bleach to cultures of microbes and observe under a microscope as it breaks down cell walls.
2. Natural Gas Combustibility
Natural gas has high flammability and thermal energy released when its hydrocarbon chains combust with oxygen. This flammability comes from the chemical structure of natural gas molecules that enables an exothermic oxidation reaction.
Over 43% of United States households rely on natural gas for cooking and heating through this chemical burning process.
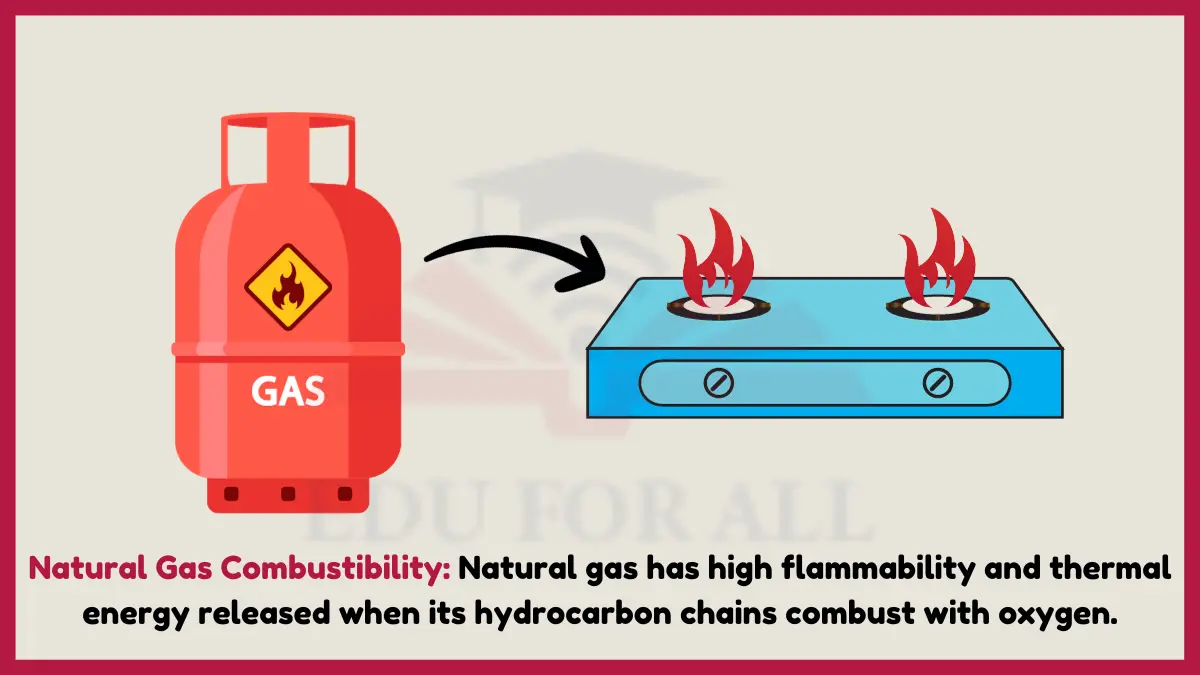
Experiment: Use a lighter to ignite a small stream of natural gas from a nozzle and observe the resulting flame.
3. Table Salt’s Solubility
Sodium chloride in table salt readily dissociates into sodium and chloride ions when dissolved in water at normal temperatures. This solubility comes from the polar nature of the ionic sodium chloride crystals that allows water to readily absorb the molecules.
On average, Americans consume about 3,400 milligrams of sodium each day.

Experiment: Add spoonfuls of salt into warm and cold water, stir and time how long it takes the salt grains to fully dissolve.
4. Nitrous Oxide’s Analgesic Properties
Nitrous oxide gas possesses pain-reducing pharmacological effects mediated through neural signaling inhibition. The chemical structure of N2O enables it to stimulate receptors that alter pain perception.
It is used as an analgesic for about 25 to 30 percent of women in labor in American hospitals.
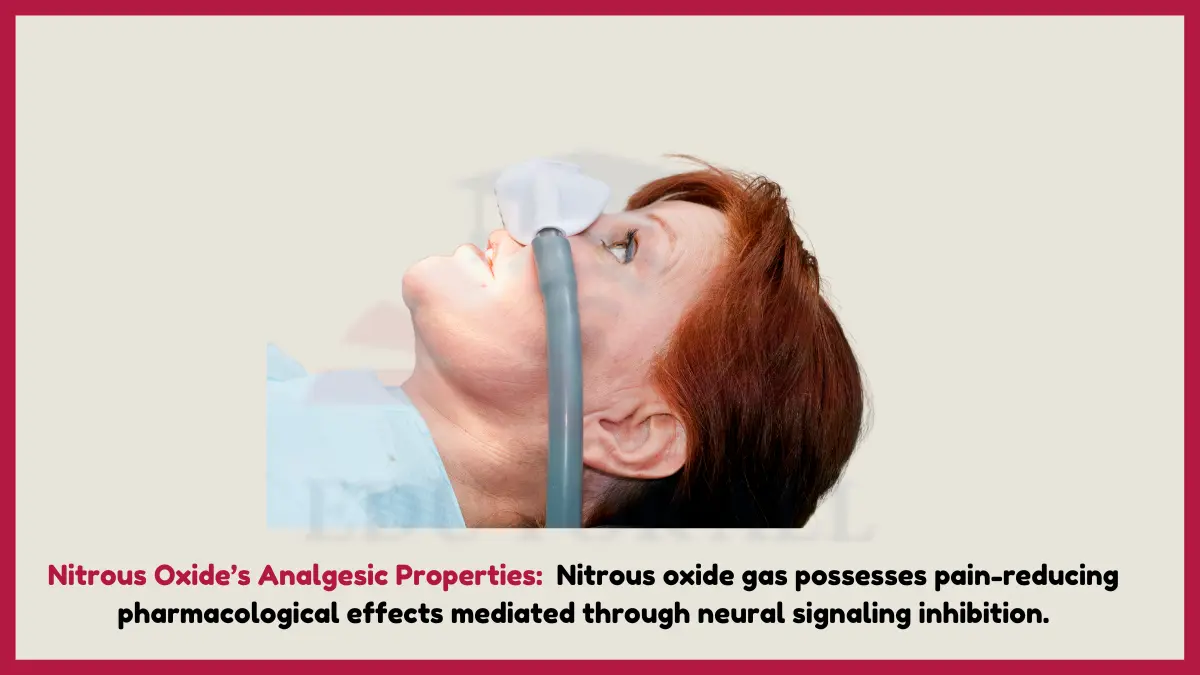
Experiment: While supervised by an instructor, briefly inhale a therapeutic dose of nitrous oxide and have a peer pinch your skin to see if it hurts less.
5. Titanium’s Resistivity to Corrosion
Titanium forms an oxidized passive layer when exposed to moisture that shields against further reactivity and corrosion. This corrosion resistance stems from the reactive chemical nature of titanium metal to readily form protective titanium oxide compounds rather than eroding further.
224,000 pounds of titanium metals are used in aerospace applications each year in the United States.
Experiment: Leave titanium and iron metal coupons in vinegar for a week and observe which sample shows more corrosion.
6. Polyester’s Wrinkle Resistance
Polyester clothing resists wrinkling due to chemical alkyl chain stiffness from repeating ester groups along its backbone even after washing. The chemical structure of polyester makes the polymers resistant to bending and deforming.
65% of all United States apparel production uses polyester material.

Experiment: Wash and dry similar-sized cotton and polyester swatches, then observe which fabric appears more wrinkled.
7. Vinegar’s Ability to Dissolve Lime Deposits
The acetic acid in vinegar reacts with calcium carbonate compounds, chemically dissolving mineral buildup from hard water over time. Vinegar is chemically able to decompose other compounds like lime due to its acidic proton donation properties.
Over 5 million gallons of white vinegar are produced yearly in the United States alone.

Experiment: Soak hard water-stained glass or utensils in vinegar and watch bubbling reaction dissolve deposits.
8. Liquids Ability to Take Shape of Containers
Liquids conform to the shape of their containers due to the capacity of independent molecules to diffuse and move past one another. This chemical trait of fluidity comes from weak intermolecular attractive forces in liquids allowing component particles to flow with ease.
The United States yearly produces over 14 billion gallons of contained liquids including fuels, solvents, and beverages.
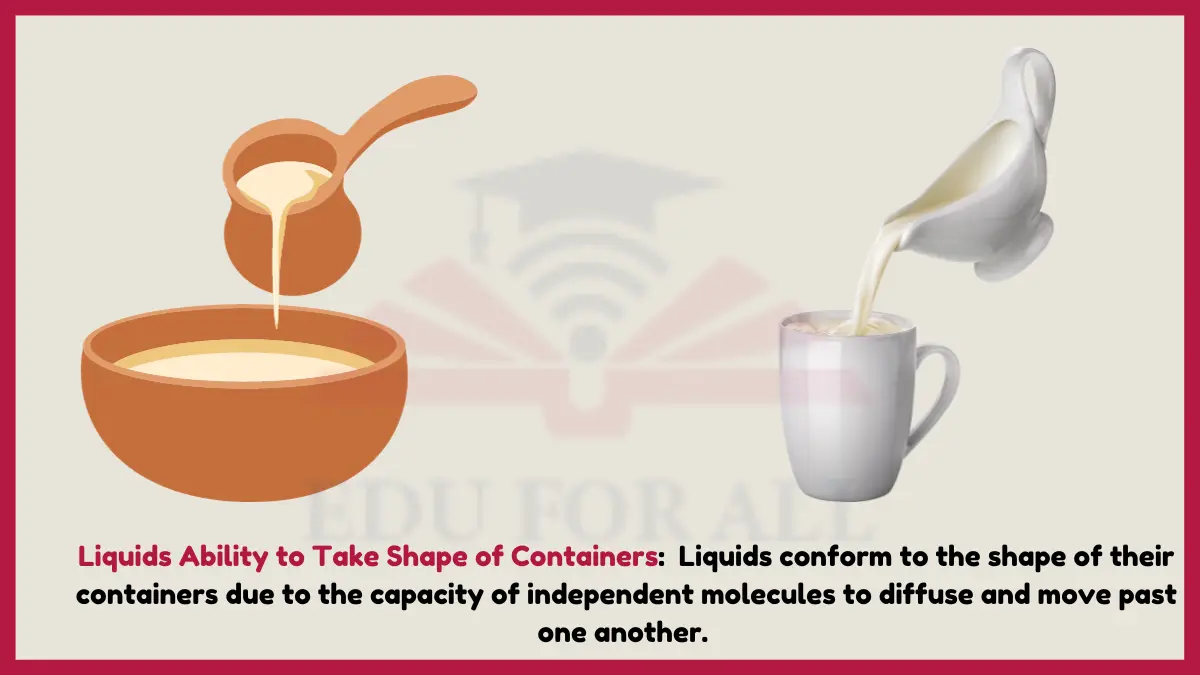
Experiment: Pour water into differently shaped containers and see it fill up the shape of each.
9. Quartz Midpoint Thermal Expansion
Fused quartz glass maintains very consistent dimensions across temperature swings due to low thermal expansion arising from strong silicon-oxide bonds. The sturdy chemical bonds between quartz molecules change length minimally in response to temperature.
Quartz glass technologies account for over $6 billion in global sales revenue on an annual basis.
Experiment: Carefully measure and record dimensions of a fused quartz piece at hot and cold temps to quantify lack of thermal expansion.
10. Activated Charcoal Odor Absorption
Activated charcoal’s porous surface has affinity to trap gaseous compounds through adsorption. This comes from an innate chemical property of the numerous pores to attract and adhere to compounds.
Over 205 million kilograms of activated charcoal are produced globally each year for filtration uses.

Experiment: Place charcoal briquettes and fresh bread separately in sealed containers then smell contents after 1-2 days to compare odor absorption.

