From the air we breathe to fuels powering society, mixtures of chemical elements that form compounds exist all around us. Familiar examples of compounds include table salt, composed of bonded sodium and chloride, water’s hydrogen and oxygen molecules, and glucose’s carbon, oxygen, and hydrogen ingredients providing metabolic energy.
Examples of Compounds
Here are some of the most common Examples of Compounds:
1. Water (H2O)
Water is comprised of two hydrogen atoms covalently bonded to a single oxygen atom in a bent formation. This polarity allows it to readily dissolve ionic compounds as well as provide an essential solvent for biological systems.

Do You Know?
Water isn’t just wet, it’s a compound of 2 hydrogens and 1 oxygen atom, bonded together in a V-shaped molecule.
Experiment: Use a battery source to carefully perform electrolysis, separating water into its components hydrogen and oxygen gases. Collect each gas through displacement into balloons and test by igniting to observe the dramatic fiery recombination into water.
2. Sodium Chloride (NaCl)
Table salt consists of a giant ionic lattice array of smaller metallic sodium cations (Na+) surrounded by larger chlorine anions (Cl-). The polarity allows table salt to dissolve into essential electrolytes in biological contexts while its savoriness drives seasoning applications.
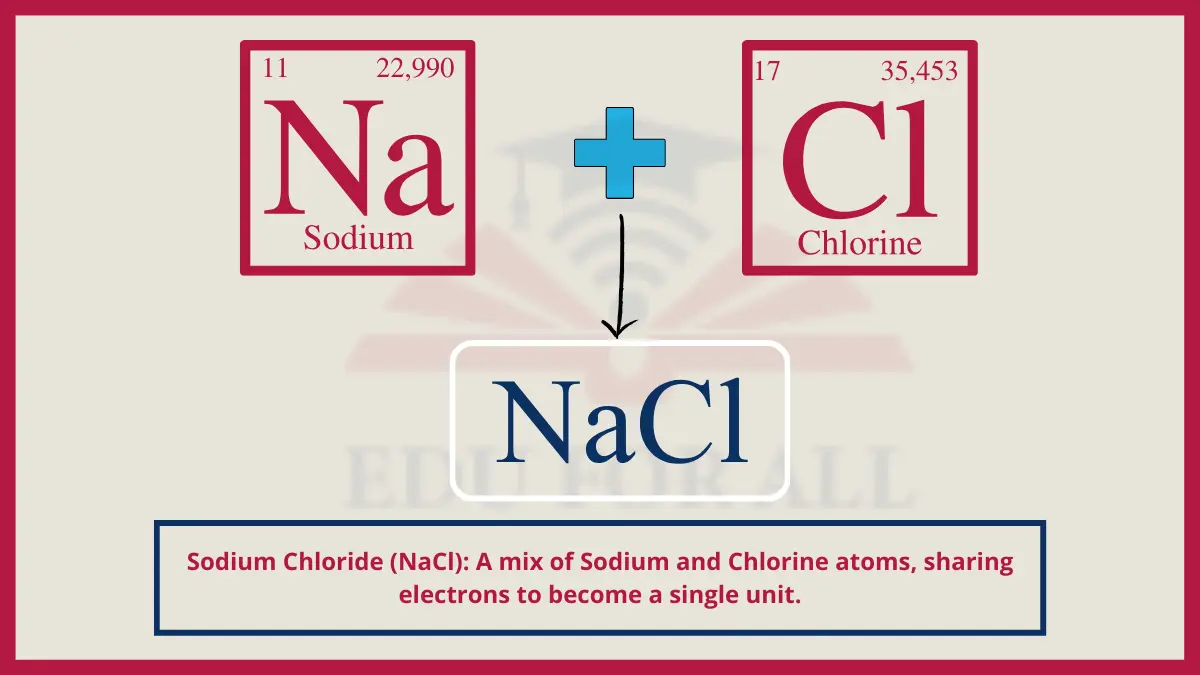
Do You Know?
Over 230 million tons of table salt is produced globally per year.
3. Sucrose (C12H22O11)
The molecular structure of sucrose sugar is a disaccharide, containing 12 carbon atoms bonded with hydrogen and oxygen in a ring formation called a glycosidic bond between glucose and fructose.
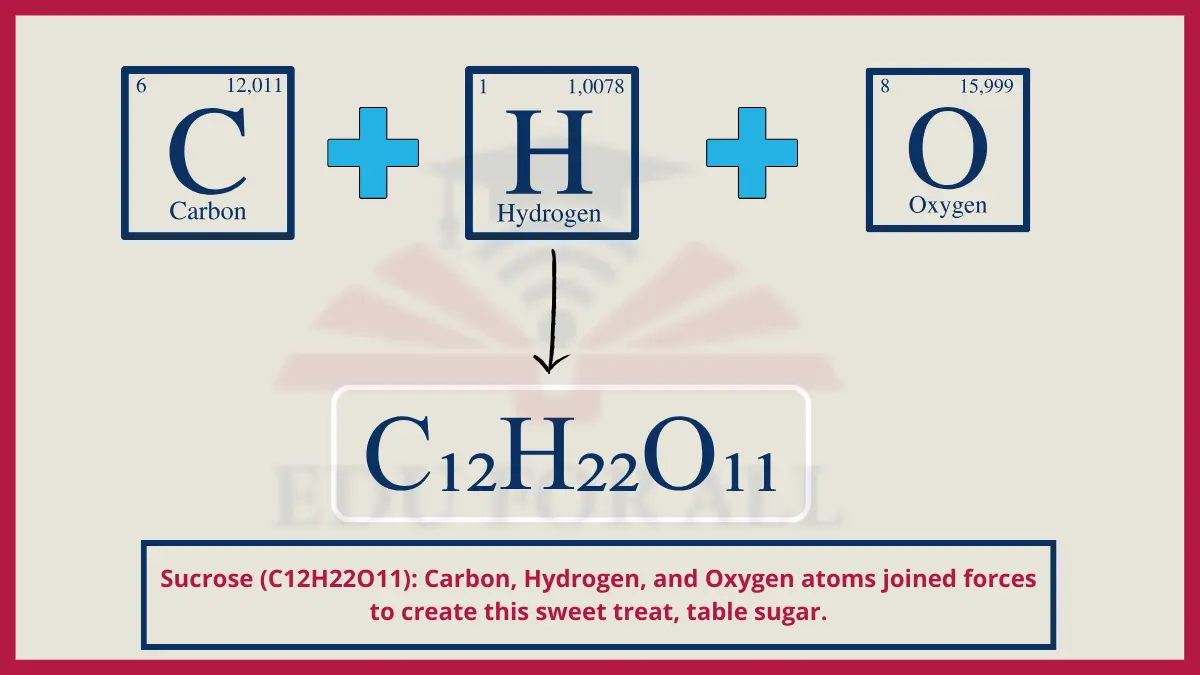
Do You Know?
The average American consumes over 20 teaspoons of added sugar daily amounting to around 66 pounds per year.
Experiment: Construct models using marshmallows and toothpicks to enable visualization of single, double, and triple covalent bonding patterns in saturated sugar compounds like sucrose. Examine their structural stability.
4. Sodium Bicarbonate (NaHCO3)
Sodium bicarbonate forms crystalline ionic structures composed of sodium cations bonded to bicarbonate anions. This polarity allows it to dissolve and act as a leavening agent in cooking.
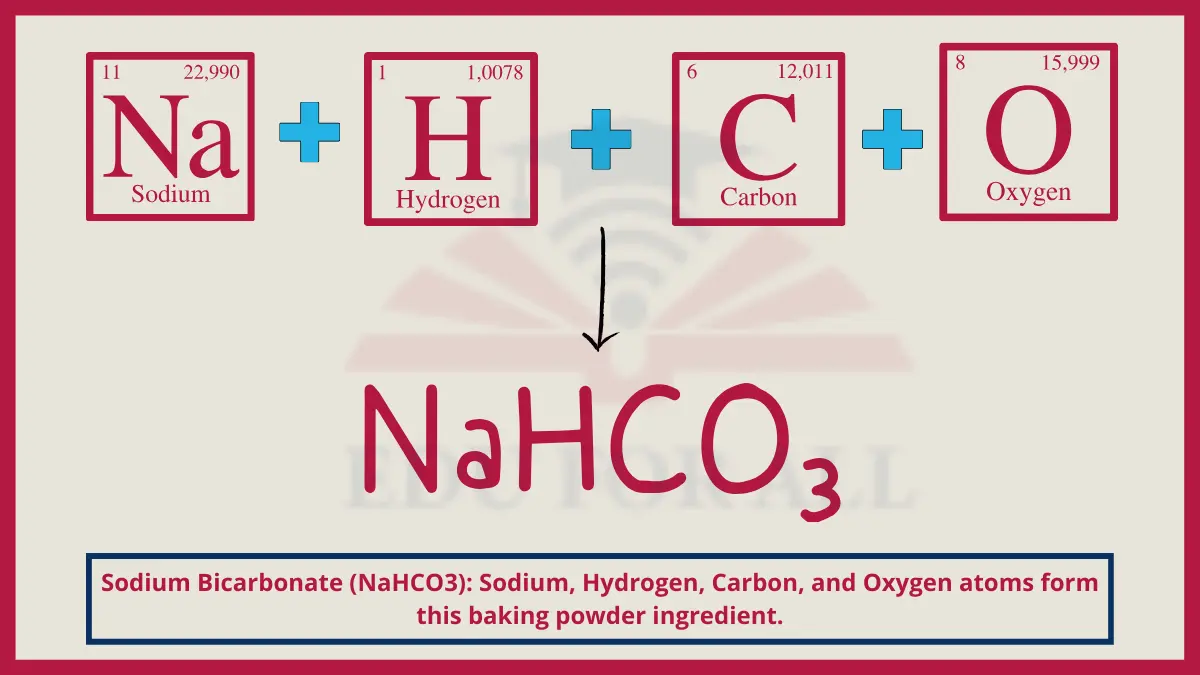
Do You Know?
Over 6 million tons of sodium bicarbonate are produced annually for applications from cooking to pharmaceuticals.
Experiment: Mix heated solutions of baking soda (sodium bicarbonate) with calcium chloride then filter out the precipitated calcium carbonate to yield pure sodium chloride table salt crystals.
5. Nitrous Oxide (N2O)
Gaseous nitrous oxide used for anesthesia consists of two tightly bonded nitrogen atoms joined to an oxygen atom in a linear formation.
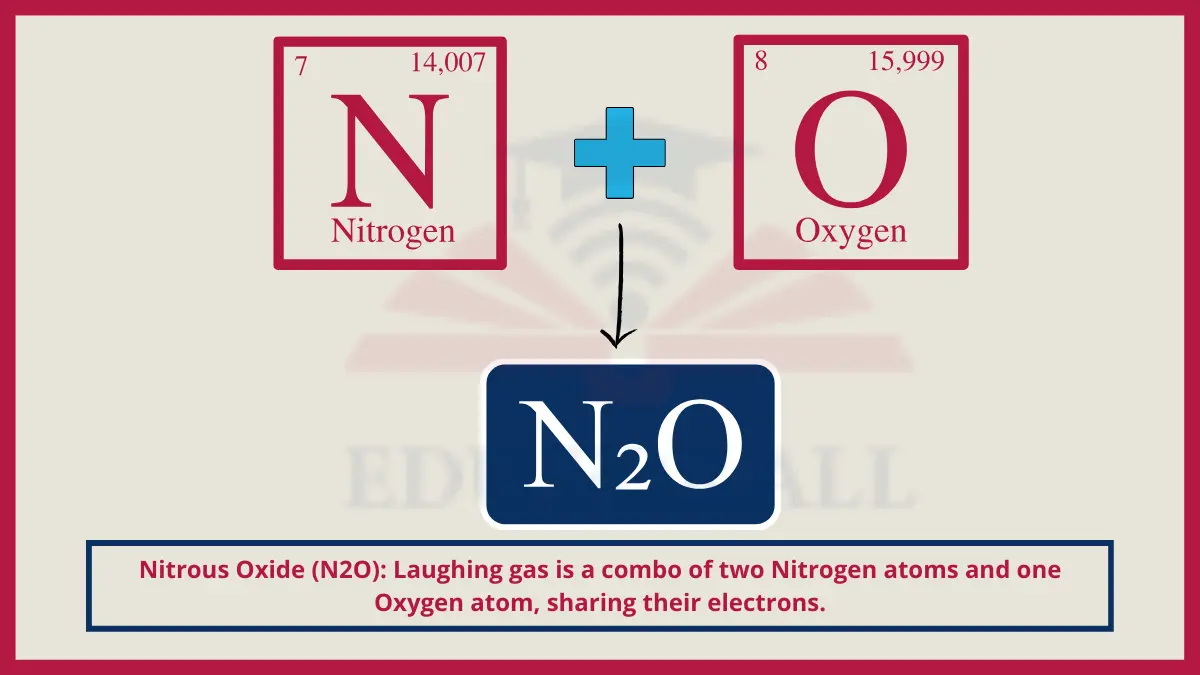
Do You Know?
As an analgesic gas, nitrous oxide is considered 25% more potent by weight than morphine is.
Experiment: React ammonium nitrate with sulfuric acid in a controlled lab setting to generate nitrous oxide gas which can be collected by displacing water for examination.
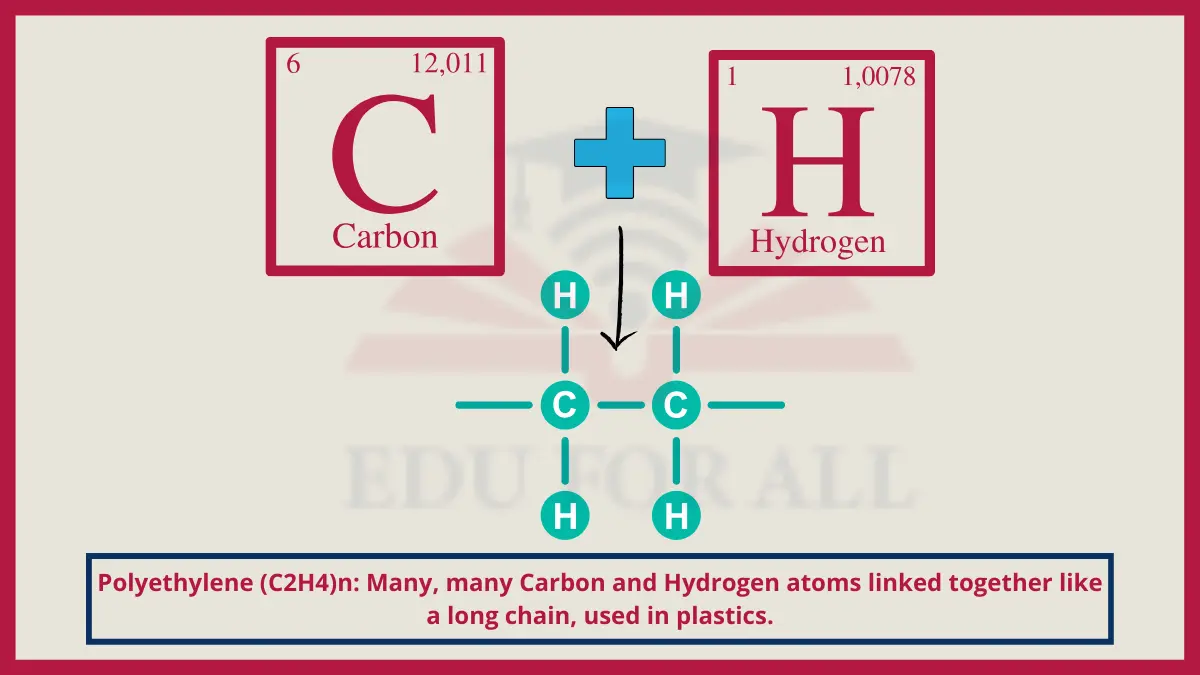
6. Polyethylene (C2H4)n
Polyethylene’s structure links many ethylene monomers into extremely long, flexible and durable polymer chains.
Do You Know?
Global plastic production has now exceeded 380 million tons annually of which polyethylene is the single largest component.
Experiment: Construct a model demonstrating polymerization using paper clips linked into a long chain. Simulate effects of cross linkage and crystallinity levels on resulting material stiffness.
7. Ammonium Nitrate (NH4NO3)
This common fertilizer is comprised of the polyatomic ammonium cation (NH4+) paired to nitrate anions (NO3-).

Do You Know?
Over 21 million tons per year of ammonium nitrate fertilizers are manufactured globally, valued at $16 billion.
Experiment: Watch crystal formation as aqueous solutions of ammonia and nitric acid are carefully mixed, then evaporate to leave ammonium nitrate solids.
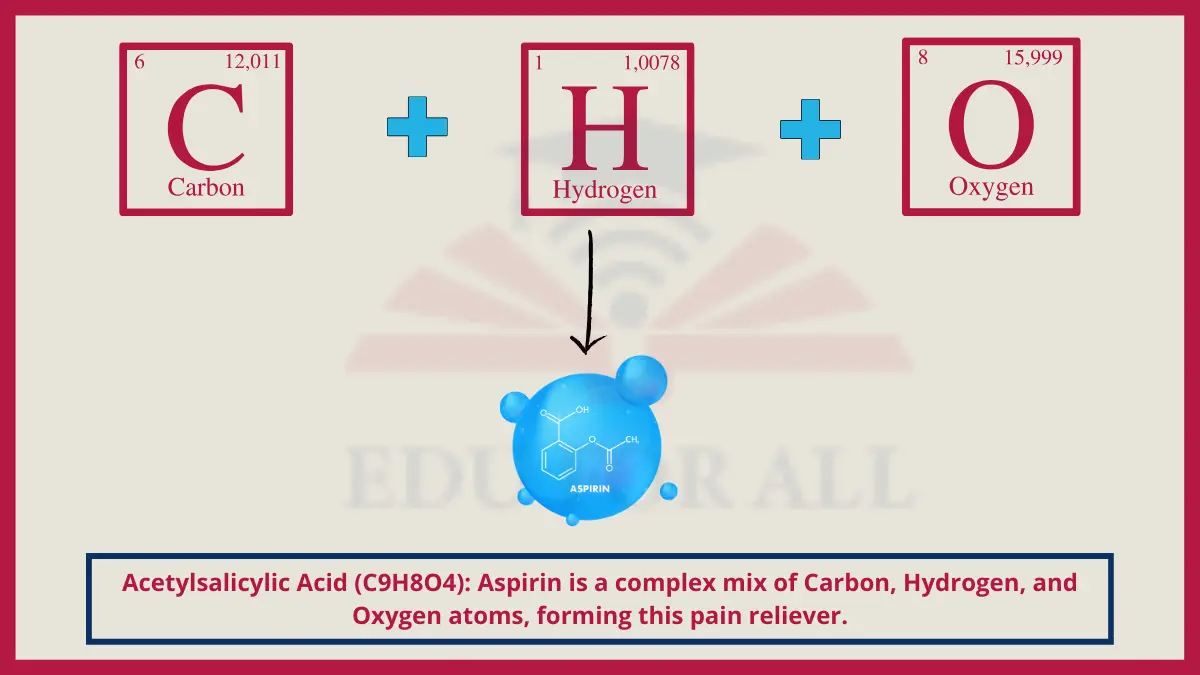
8. Acetylsalicylic Acid (C9H8O4)
The compound commonly known as aspirin has the chemical name acetylsalicylic acid. Its functional groups thin blood while reducing inflammation signals.
Do You Know?
An estimated 50,000 tons of aspirin are produced and consumed globally per year.
Experiment: Synthesize aspirin by carefully reacting salicylic acid with acetic anhydride using sulfuric acid as an acid catalyst while wearing gloves, goggles, and mask.
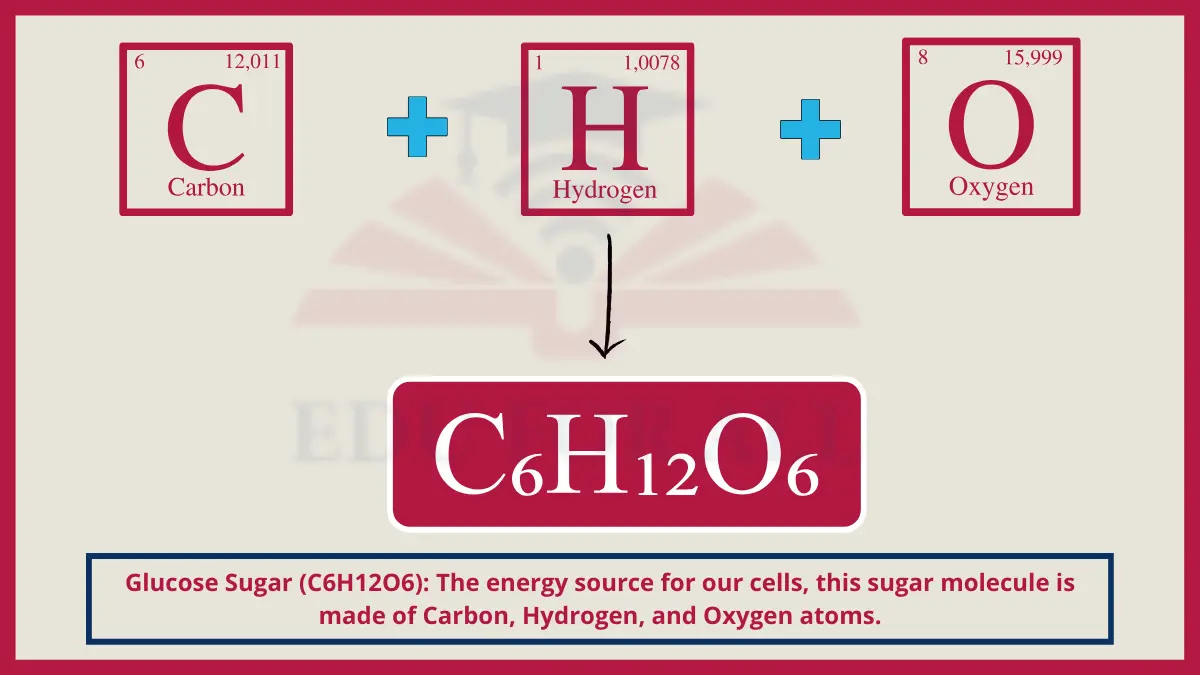
9. Glucose Sugar (C6H12O6)
Glucose, a simple monosaccharide sugar, contains 6 carbon atoms double bonded to 5 hydroxyl groups and a single hydrogen atom per its molecular structure.
Do You Know?
The average person metabolically processes about 160 grams of glucose sugar each day, making it a prime cellular energy source.
Experiment: Test reactions with glucose solution using Benedict’s indicator under heat then compare colors against a reference chart to quantify concentration.
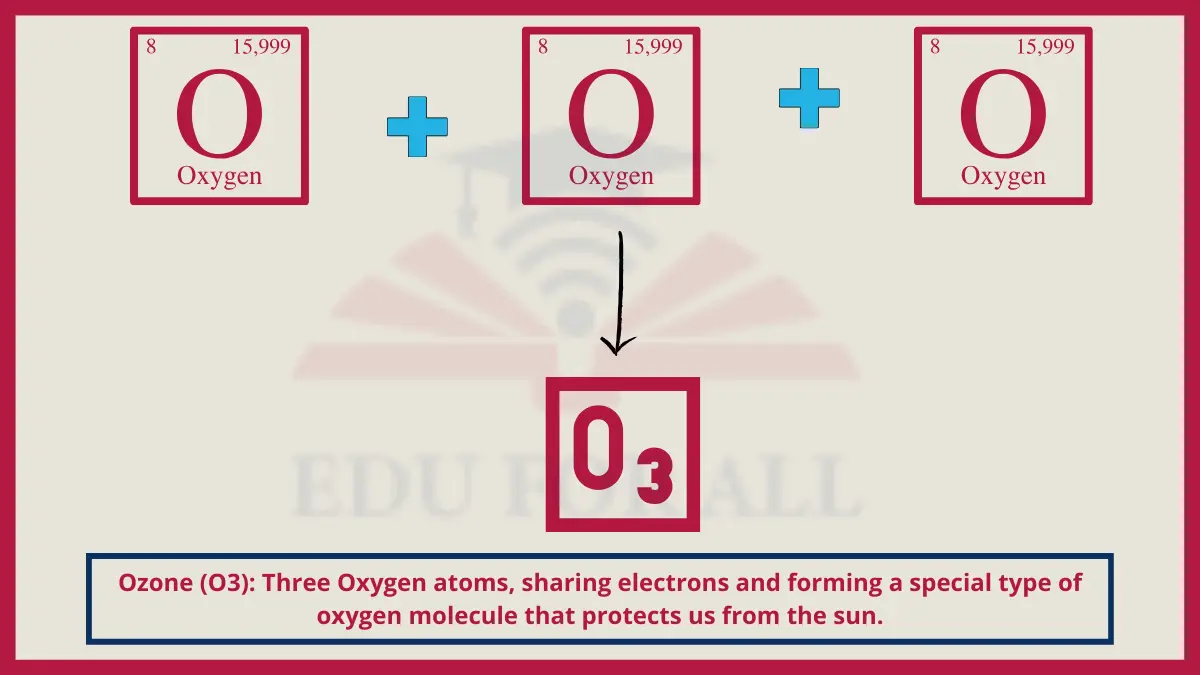
10. Ozone (O3)
Ozone consists of three bound oxygen atoms instead of the usual O2 pair. This chemical instability drives oxidative planet-shielding capacity but also constitutes ground pollution.
Do You Know?
About 90% of atmospheric ozone resides in the ozone layer about 9-18 miles up but is depleted by CFCs and other pollutants.
Experiment: Use tubing, electricity and various oxidation catalysts to generate ozone gas from oxygen, detect its sharp chlorine-like smell at low concentrations, then measure how long atmospheric half-life lasts.

