Entropy surrounds us in many forms in daily life. Classic examples of entropy include diffusion processes like perfume dispersing from a bottle until molecules are randomly distributed in a room. Other everyday instances are ice cubes melting into disordered liquid water puddles, iron rusting over time into degraded chemicals, or smoke irreversibly mixing with air.
We also see entropy in operation when making coffee – cream combines with coffee until thoroughly blended. Food rotting or leaves decomposing shows increasing molecular disorder breaking complex structures down. Even a sandcastle losing shape as it returns to an amorphous pile demonstrates entropy increasing randomness.

Understanding Entropy
Imagine your room. When you wake up, it’s tidy – clothes folded, toys put away. That’s low entropy, like a neat card deck. But as you play, things get messy – clothes on the floor, toys scattered. That’s entropy increasing, like shuffling the deck. Every day, things go from tidy to messy, because that’s what entropy loves to do.
Examples of Entropy
Here are examples of entropy in everyday life:
1: Diffusion of perfume molecules
When you spray perfume, the perfume molecules diffuse from areas of high concentration (the nozzle) to low concentration around the room. This spreading out and dispersing of molecules is an increase in disorder and entropy.
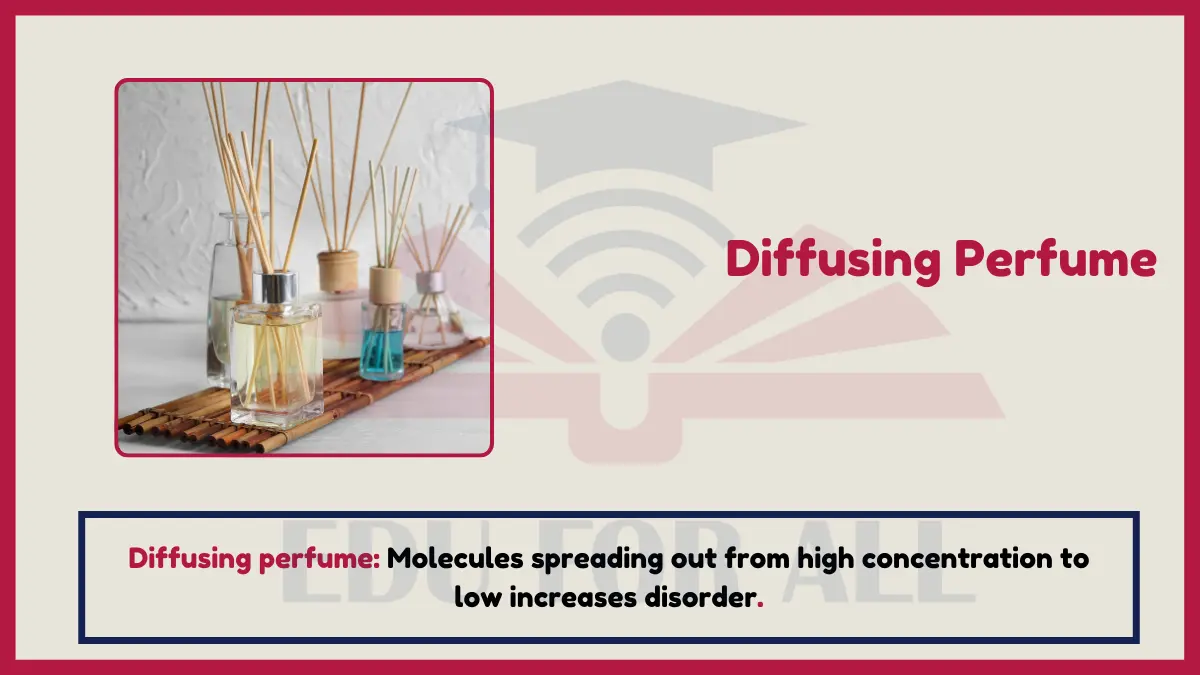
Experiment: Spray perfume on one part of a table. Time how long it takes to smell the perfume in other areas. The molecules diffuse to increase entropy.
2: Mixing of cream into coffee
When you pour cream into black coffee, the cream spreads out and disperses into the coffee as the colors mix together. This mixing process increases the randomness and disorder in the cup, thereby increasing entropy.

Experiment: Record how long it takes for poured cream to fully mix into a cup of black coffee. The entropy rapidly increases from the highly ordered separated liquids to the well-mixed random distribution of coffee and cream.
3: Rusting of iron
As iron rusts over time when exposed to oxygen and moisture, the solid iron reacts and degrades into rust, a chemical conversion that increases disorder at the molecular level. Rust takes up more volume than iron also increasing entropy.

Experiment: Take two same size iron nails, expose one to air and moisture, observe, and compare rust formation over time. The rusting nail has increasing disorder and entropy.
4: Melting of ice cubes
When an ice cube melts, the water molecules transition from the highly ordered crystalline solid ice structure to a more disordered liquid water arrangement spread over a larger volume and area. This thermodynamic process increases entropy.

Experiment: Record how long it takes for an ice cube to fully melt at room temperature. The phase change from solid to liquid causes a marked increase in entropy.
5: Smoke and gases dispersing
Smoke or gases released into air spread out and mix with their surroundings in an irreversible process indicating a rise in entropy to a state of heightened disorder and randomness.

Experiment: Record the visible trail of smoke from an incense stick. As the smoke diffuses into the room disorder rapidly increases.
6: Food rotting
The decomposition and rotting of food via mold, bacteria, and chemical processes reflects irreversible molecular disorder as complex organic materials break down into simpler inorganic forms and gases. The molecules become disordered through digestion and decay.

Experiment: Monitor the visual changes over one week time for a rotting fruit or vegetable. The rotting process involves increased molecular randomness.
7: Tree leaves decomposition
As leaves fall from trees and slowly break down on the forest floor from organisms and weathering, their organized biological structures transform into disarranged simpler organic and inorganic components through this irreversible process driven by increasing entropy.

Experiment: Collect fallen leaves, soak half in water, photograph the faster decay over time of the soaked leaves. Record the increased disorder visually over several weeks time.
8: Sandcastle collapsing
The collapse of an ordered sandcastle shape back into a flattened shapeless pile of sand grains demonstrates the natural tendency toward higher entropy or increasing randomness from order moving to disorder. Less energy is required to maintain the shapeless sand pile.
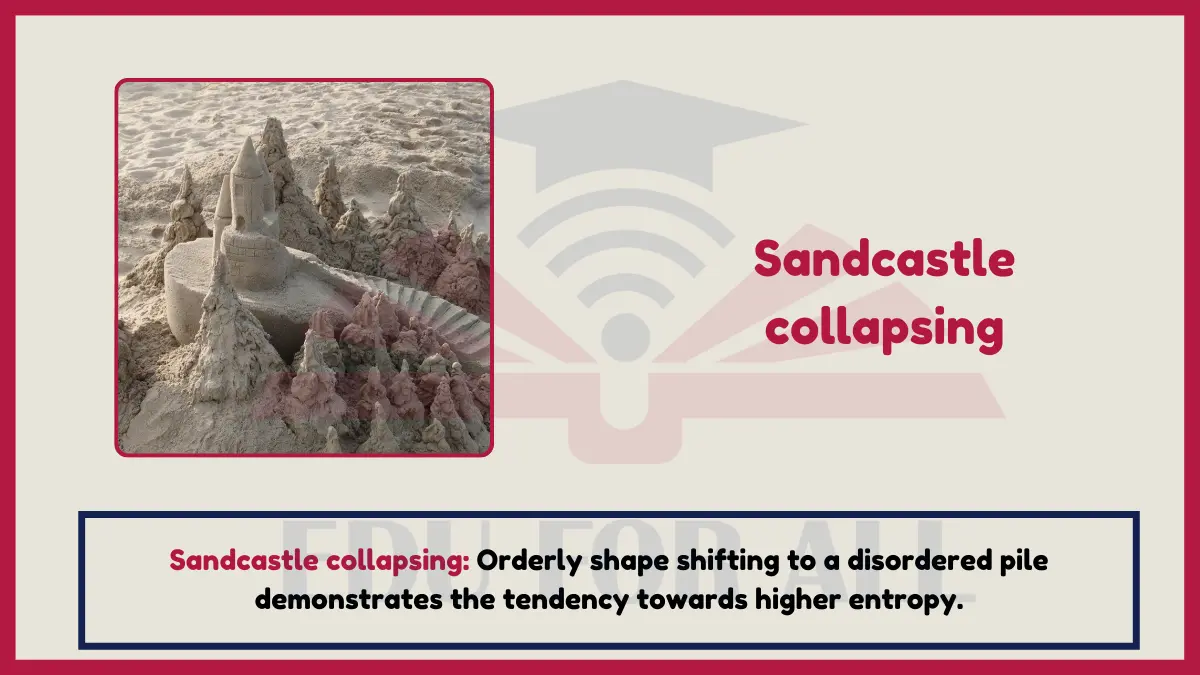
Experiment: Use water to quickly enable a sandcastle to degrade into a pile which better allows particles to become disordered. Observe the nature of increasing entropy.
9: Mixing paint colors
Mixing bottles of paint together causes pigment particles to spread out, blend together, and become more disorganized in arrangement, indicating a rise in entropy enabled by the irreversible interactions of color mixtures.

Experiment: Take bottles of red, blue and yellow paint. Mix pairs together recording how long it takes to achieve a uniform new color showing entropy rise.
10: Wind blowing leaves into disorder
As wind blows through an orderly pile of raked leaves, it forces the leaves into random unpredictable scattered orientations demonstrating entropy as the leaves transform from order into heightened disorder in their positions.

Experiment: Rake a neat pile of leaves, use a fan to blow air into the pile and record the increasing disordered distributions. The irreversible reorientation reflects entropy.

